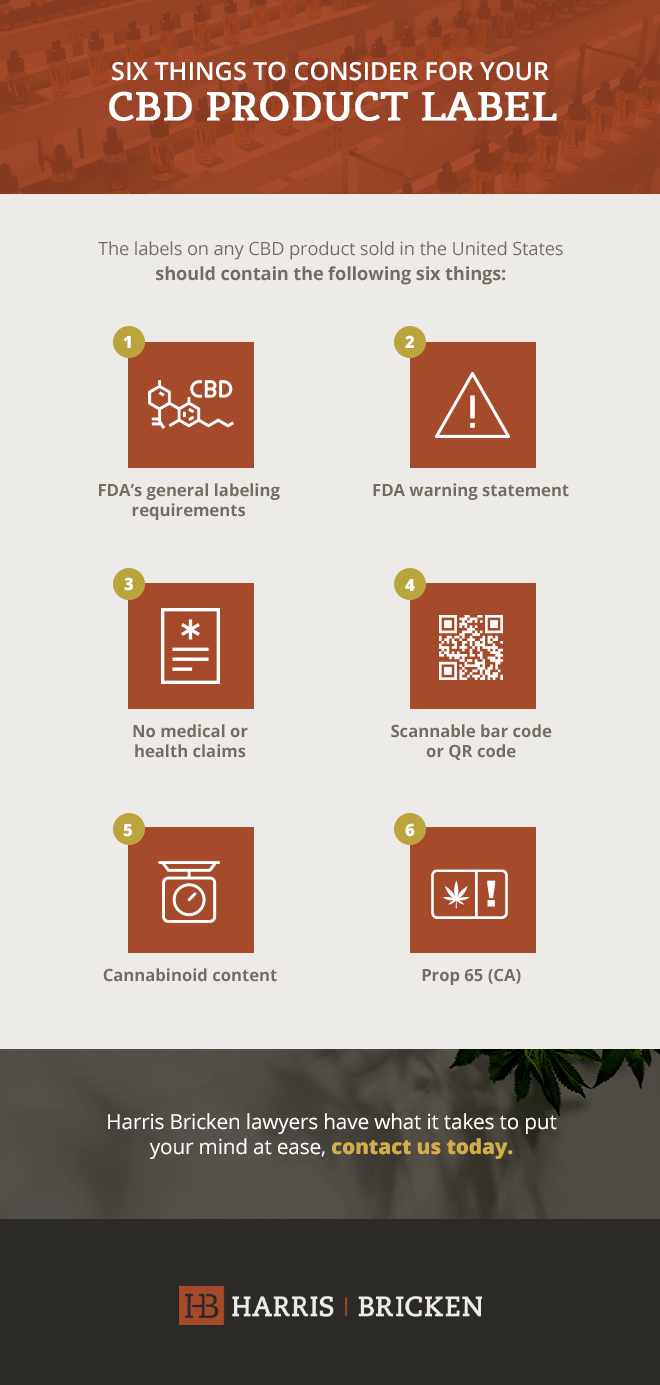
39 information that must be lawfully provided on food labels
Application for Permanent Residence – Business Immigration Program ... Information on medical instructions will be provided to you by the IRCC office. When you receive your assessment notice you will also receive medical forms for yourself (and any dependants, if applicable ) and instructions on how to access a list of doctors in your area who are authorized to conduct immigration medical examinations (see below). Regulatory Consulting Services- I3CGLOBAL FDA Label review (Food, Drugs, Cosmetics) means verification of existing labels by our experts against FDA’s labeling regulation READ MORE FDA Agents are the primary contact point person or company between the foreign facility and the FDA in case of any emergency and routine registration matters.
› scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Mar 29, 2022 · A reference to information submitted previously must identify the file by name, reference number, volume, and page number where the information can be found. A reference to information submitted to the agency by a person other than the sponsor is required to contain a written statement that authorizes the reference and that is signed by the ...

Information that must be lawfully provided on food labels
CFR - Code of Federal Regulations Title 21 - Food and Drug … 29.3.2022 · A brief statement of any other information that would aid evaluation of the proposed clinical investigations with respect to their safety or their design and potential as controlled clinical trials to support marketing of the drug. (11) Relevant information. If requested by FDA, any other relevant information needed for review of the application. Membership Information and Guidelines | The Bay Club For detailed information regarding the booking guidelines for your membership, click on the Guidelines link on the booking site’s navigation bar. Court Check-In. All members must check in at the Front Desk or Court Reservations Desk for their court assignment prior to play or be subject to the Late Cancellation fee of $25. Herbicide Food BASF United States ... MUST HAVE ALL LABELING APPLICABLE TO YOUR LOCATION IN YOUR POSSESSION FOR THIS PRODUCT TO BE LAWFULLY APPLIED AFTER 03/15/2022. www ...
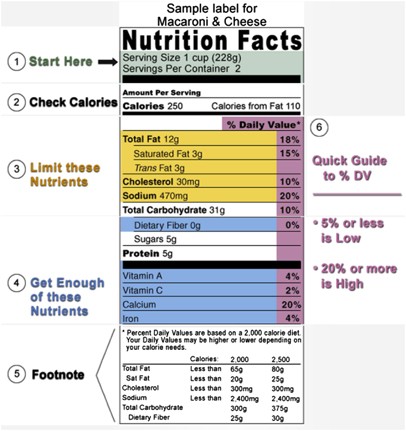
Information that must be lawfully provided on food labels. Part 552 - Solicitation Provisions and Contract Clauses (a) General.When a GSAR provision or clause is used without deviation in a solicitation or contract, it shall be identified by number, title, and date ( e.g., 552.211-77, Packing List (FEB 1996)). (b) Deviations. (1) Federal Acquisition Regulation deviations.When a GSAR provision or clause is used with an authorized deviation in lieu of a FAR provision or clause in a solicitation … Engenia Herbicide As an over-the-top dicamba with the lowest use rate, Engenia ® herbicide helps you cover more acres more efficiently , so you can focus on tackling more items on your to-do list this season. Powered by BASF's proprietary BAPMA salt and high-performance dicamba, you can be confident that you're getting the most advanced herbicide available for dicamba-tolerant soybeans. NYS Pharmacy:Laws, Rules & Regulations:Part 63 - New York State ... 20.8.2009 · §63.3 Licensing examinations. Effective January 1, 2022, each candidate applying for licensure as a pharmacist in New York State shall pass an examination or examinations acceptable to the board of pharmacy for licensure purposes and determined by the department to be satisfactory for measuring the applicant's knowledge regarding the curricular areas defined … › Programs › CEHIHCPFAQS - California For example, food product labels must comply with Title 21, CFR Part 101 – Food Labeling. Required information on food labels include a statement of identity, ingredient list in descending order of predominance by weight, net quantity of product in the package, an address for the responsible party and a nutrition facts panel, when applicable.
› news-events › public-health-focusFDA Regulation of Cannabis and Cannabis-Derived Products ... In addition, under 21 CFR 530.20(b)(2), if scientific information on the human food safety aspect of the use of the approved human drug in food-producing animals is not available, the veterinarian ... Enforcement – Welcome to the City of Fort Worth Call 817-392-1234 and provide the following information: address and description of animal; time of incident; other pertinent details; You will be advised to maintain a complaint log for 7 to 10 days beginning with the date that the complaint is filed. The information in the log must contain the date, times and duration of the noise nuisance. › membershipguidelinesMembership Information and Guidelines | The Bay Club Cell phone use is permitted only in the Main Lobby, Food & Beverage, and outdoor areas. Please refrain from conversing on speaker phone or FaceTime in public gathering spaces. Silent cell phone use (no phone calls) only is permitted in the Fitness Center and Clubhouses. Ringers must be off on the golf course, driving range, tennis and squash ... › current › title-21eCFR :: 21 CFR Part 812 -- Investigational Device Exemptions When the sponsor receives from the IRB information concerning the public disclosures under § 50.24(a)(7)(ii) and (a)(7)(iii) of this chapter, the sponsor shall promptly submit to the IDE file and to Docket Number 95S-0158 in the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD ...
IHCPFAQS - California AB 45 provided the regulatory framework for industrial hemp and its derivatives in specific ... For example, food product labels must comply with Title 21, CFR Part 101 – Food Labeling. Required information on food labels include a statement of identity, ingredient list in descending order of predominance by weight, net quantity of ... eCFR :: 21 CFR Part 812 -- Investigational Device Exemptions When the sponsor receives from the IRB information concerning the public disclosures under § 50.24(a)(7)(ii) and (a)(7)(iii) of this chapter, the sponsor shall promptly submit to the IDE file and to Docket Number 95S-0158 in the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852, copies of the information … World-Class Regulatory Consulting Services- I3CGLOBAL EXCEPTIONAL SERVICE ONE STOP FOR ALL YOUR REGULATORY NEEDS Fully functional service lined up for INDIA, EU & USFDA in one place. CONTACT US A trustworth company SUSTAINABLE PARTNER for leading corporates, medium and small scale healthcare product manufacturers across the world. FOR EU AND USFDA CONTACT US PROVEN PRACTICES … Herbicide Food BASF United States ... MUST HAVE ALL LABELING APPLICABLE TO YOUR LOCATION IN YOUR POSSESSION FOR THIS PRODUCT TO BE LAWFULLY APPLIED AFTER 03/15/2022. www ...
Membership Information and Guidelines | The Bay Club For detailed information regarding the booking guidelines for your membership, click on the Guidelines link on the booking site’s navigation bar. Court Check-In. All members must check in at the Front Desk or Court Reservations Desk for their court assignment prior to play or be subject to the Late Cancellation fee of $25.
CFR - Code of Federal Regulations Title 21 - Food and Drug … 29.3.2022 · A brief statement of any other information that would aid evaluation of the proposed clinical investigations with respect to their safety or their design and potential as controlled clinical trials to support marketing of the drug. (11) Relevant information. If requested by FDA, any other relevant information needed for review of the application.




















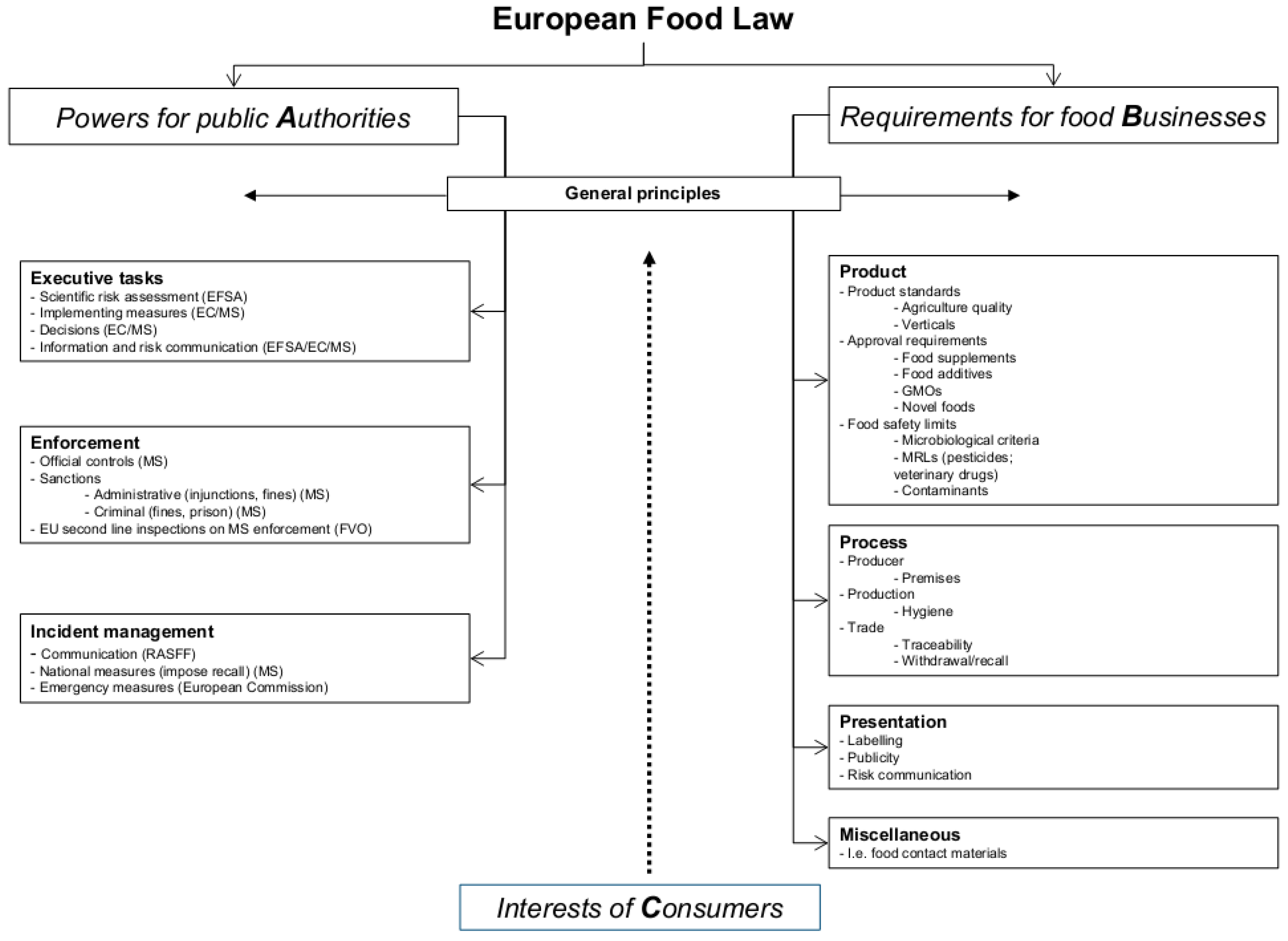

Post a Comment for "39 information that must be lawfully provided on food labels"